Christopher Damman, University of Washington
Wegovy, Ozempic and Mounjaro are weight loss and diabetes drugs that have made quite a splash in health news. They target regulatory pathways involved in both obesity and diabetes and are widely considered breakthroughs for weight loss and blood sugar control.
But do these drugs point toward a root cause of metabolic disease? What inspired their development in the first place?
It turns out your body produces natural versions of these drugs – also known as incretin hormones – in your gut. It may not be surprising that nutrients in food help regulate these hormones. But it may intrigue you to know that the trillions of microbes in your gut are key for orchestrating this process.
I am a gastroenterologist at the University of Washington who studies how food and your gut microbiome affect health and disease. Here’s an inside-out perspective on the role natural gut hormones and healthy food play in metabolism and weight loss.
A broken gut
Specialized bacteria in your lower gut take the components of food you can’t digest like fiber and polyphenols – the elements of plants that are removed in many processed foods – and transforms them into molecules that stimulate hormones to control your appetite and metabolism. These include GLP-1, a natural version of Wegovy and Ozempic.
GLP-1 and other hormones like PYY help regulate blood sugar through the pancreas. They also tell your brain that you’ve had enough to eat and your stomach and intestines to slow the movement of food along the digestive tract to allow for digestion. This system even has a name: the colonic brake.
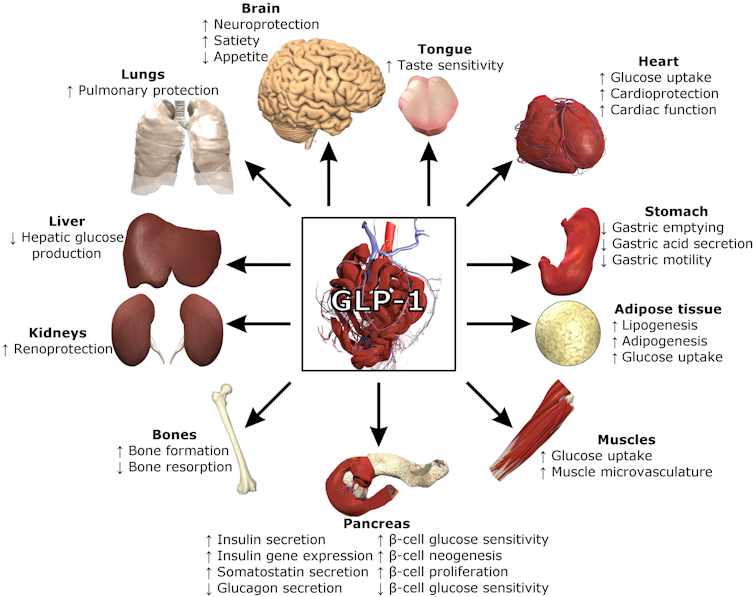
Prior to modern processed foods, metabolic regulatory pathways were under the direction of a diverse healthy gut microbiome that used these hormones to naturally regulate your metabolism and appetite. However, food processing, aimed at improving shelf stability and enhancing taste, removes the bioactive molecules like fiber and polyphenols that help regulate this system.
Removal of these key food components and the resulting decrease in gut microbiome diversity may be an important factor contributing to the rise in obesity and diabetes.
A short track to metabolic health
Wegovy and Ozempic reinvigorate the colonic brake downstream of food and microbes with molecules similar to GLP-1. Researchers have demonstrated their effectiveness at weight loss and blood sugar control.
Mounjaro has gone a step further and combined GLP-1 with a second hormone analogue derived from the upper gut called GIP, and studies are showing this combination therapy to be even more effective at promoting weight loss than GLP-1-only therapies like Wegovy and Ozempic.
These drugs complement other measures like gastric bypass surgery that are used in the most extreme cases of metabolic disease. These surgeries may in part work much like Wegovy and Ozempic by bypassing digestion in segments of the gastrointestinal tract and bathing your gut microbes in less digested food. This awakens the microbes to stimulate your gut cells to produce GLP-1 and PYY, effectively regulating appetite and metabolism.
Many patients have seen significant improvements to not only their weight and blood glucose but also reductions in important cardiovascular outcomes like strokes and heart attacks. Medical guidelines support the use of new incretin-based medications like Wegovy, Ozempic and Mounjaro to manage the interrelated metabolic conditions of diabetes, obesity and cardiovascular disease.
Considering the effects incretin-based medications have on the brain and cravings, medical researchers are also evaluating their potential to treat nonmetabolic conditions like alcohol abuse, drug addiction and depression.
A near-magic bullet – for the right folks
Despite the success and prospect of these drugs to help populations that may benefit most from them, current prescribing practices have raised some questions. Should people who are only a little overweight use these drugs? What are the risks of prescribing these drugs to children and adolescents for lifelong weight management?

While incretin-based therapies seem close to magic bullets, they are not without gastrointestinal side effects like nausea, vomiting, diarrhea and constipation. These symptoms are related to how the drugs work to slow the gastrointestinal tract. Other more severe, but rare, side effects include pancreatitis and irreversible gastroparesis, or inflammation of the pancreas and stomach paralysis.
These drugs can also lead to a loss of healthy lean muscle mass in addition to fat, particularly in the absence of exercise. Significant weight gain after stopping the drugs raises further questions about long-term effects and whether it’s possible to transition back to using only lifestyle measures to manage weight.
All roads lead to lifestyle
Despite our greatest aspirations for quick fixes, it’s very possible that a healthy lifestyle remains the most important way to manage metabolic disease and overall health. This includes regular exercise, stress management, sleep, getting outdoors and a balanced diet.
For the majority of the population who don’t yet have obesity or diabetes, restarting the gut’s built-in appetite and metabolism control by reintroducing whole foods and awaking the gut microbiome may be the best approach to promote healthy metabolism.
Adding minimally processed foods back to your diet, and specifically those replete in fiber and polyphenols like flavonoids and carotenoids, can play an important and complementary role to help address the epidemic of obesity and metabolic disease at one of its deepest roots.
Christopher Damman, Associate Professor of Gastroenterology, School of Medicine, University of Washington
This article is republished from The Conversation under a Creative Commons license. Read the original article.